Background: Myelodysplastic syndromes (MDS) are characterized by aberrant bone-marrow (BM) morphology leading to a clonal impairment of hematopoiesis. Worsening BM failure (BMF) and increasing myeloblasts are observed as the predominant consequences of MDS disease progression from very low risk (VLR) to higher risk (HR) to acute myeloid leukemia (AML). Patients are stratified based on the degree of BMF and risk of AML transformation to help guide treatment decisions. Approximately 30% of MDS patients exhibit various autoimmune diseases that can include autoantibodies targeting neutrophils, erythrocytes, and/or platelets. Autoimmune phenomena play an important role in MDS yet are poorly characterized. We hypothesized that early in LR-MDS, dysregulated interactions between stromal cells and developing Natural Killer (NK)/T-cells promote rapid/unregulated development resulting in autoreactivity ( Fig. 1A). We predict dual effector mechanisms: CD16-mediated antibody-dependent cellular cytotoxicity (ADCC) and loss of HLA class I (HLA-I) expression by developing hematopoietic lineage cells making them targets for “missing-self” reactivity ( Fig. 1A).
NK cells are important sentinel cells that recognize tumors and dysfunctional cells that lose expression of HLA class I. Such surveillance is guided by interactions between NK inhibitory receptors and HLA class I ligands that ‘train’ NK cells to distinguish diseased cells, exhibiting perturbed HLA expression, from healthy cells with normal expression. The effects of these interactions combined with expression of activating receptors (e.g. CD16, NKG2D, DNAM1, FasL, TRAIL) allow NK cells to engage a target cell through many activating mechanisms, importantly including antibodies eliciting an antibody-dependent cellular cytotoxicity (ADCC) reaction.
Autoantibodies are well-established in MDS, yet no one has profiled whether they may trigger ADCC/complement. Further, no one has ever thoroughly characterized BMMCs from LR-MDS patients before. In our study, we observed a clear link between signatures of bone marrow failure at LR-MDS and a significant increase in BM CD57+NK and CD8 T cells expressing a highly activated and dysregulated phenotype.
Method: Ex-vivo profiling of 19 MDS patients (N=10 LR/IR-MDS, N=9 HR-MDS) BM mononuclear cells (BMMCs) was performed by mass cytometry (CyTOF)( Fig.1B). Cells were stained with three different antibody panels each consisting of 44 antibodies focused on: specific markers of hematopoiesis and myeloid or lymphoid cell lineages; specific markers of development, effector function, homing, exhaustion, and T-cell memory and proliferation; expression of HLA class I and activating ligands which are critical regulators of NK inhibition.
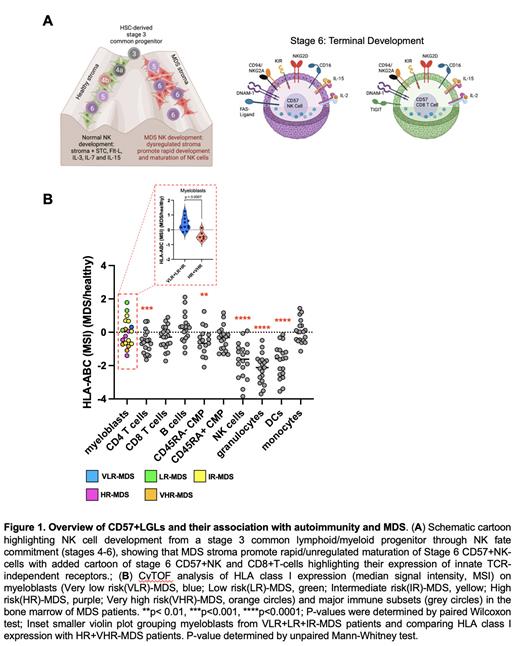
Results: In our study we observed that a major fraction of MDS patients present with clonal-like expansions of BM-derived CD57+CD8+T-cells and natural killer (NK) cells that strongly correlate with autoimmune-cytopenias and BMF-enriched in LR-MDS (Fig.#). We observed that NK-cells and non-classical T-cells were significantly expanded (>2-fold) in LR MDS compared to HR-MDS and healthy-control BM (p<0.01). These non-classical CD57+CD8+T-cells co-expressed HLA-I-reactive and inhibitory NKG2A/KIR receptors as well as activating CD16, NKG2C, NKG2D, NKp46, TRAIL, FasL. In cancers, these cells use innate-like, TCR-independent mechanisms (traditionally ascribed to NK-cells) to target HLA-I-deficient tumor cells. Strikingly, HLA-I was significantly elevated on myeloblasts in LR-MDS compared to HR-MDS (p=0.0007)( Fig.1B), demonstrating NK-evasion strategies, but strongly reduced on dendritic cells, granulocytes, and CD4+T-cells, suggesting susceptible immune targets for destruction by NK-cells (and innate-like CD8+T-cells) (p< 0.0001) ( Fig.1B). BM-derived CD57+NK and CD8+T-cells all co-express very high levels of CD16 making them particularly ADCC reactive.
Conclusion: We identified novel innate-like CD8+CD57+ T-cell and NK-cell populations that may play a role in LR-MDS disease pathogenesis. We predict that a strategy to target these cells could help restore hematopoiesis early in the disease state. This may also reduce pressure on myeloblasts to increase HLA-I, permitting improved immune surveillance to control the blast burden and prevention MDS/AML progression. Further experiments are ongoing to validate our observations.
OffLabel Disclosure:
Feld:Syros: Research Funding; Gilead: Consultancy; Oryzon: Research Funding; Taiho: Research Funding.
5'Azacitidine as a demethylating agent

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal